Geothermal Secrets: Unlocking Earth's Energy with Fluid Analysis

Introduction to Geothermal Fluids
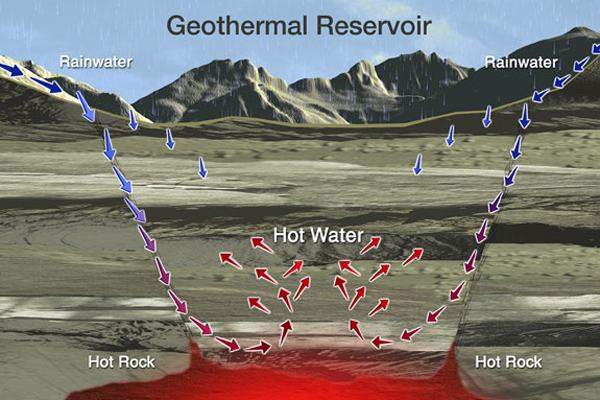
Geothermal energy, a renewable resource harnessed from Earth's internal heat, manifests in various forms, often associated with hydrothermal systems. These systems are characterized by the circulation of geothermal fluids, which are essentially heated groundwater that has interacted with the surrounding rock. Understanding these fluids is crucial for assessing the potential of geothermal reservoirs and for optimizing the extraction of geothermal energy. The study of geothermal fluids involves analyzing their chemistry, tracing their origins, and applying chemical geothermometers to estimate subsurface temperatures. This endeavor falls under the broader scope of fluid geochemistry and plays a significant role in geothermal exploration. Furthermore, the analysis of these fluids has implications for environmental geochemistry, allowing us to understand the natural release of elements and compounds from the Earth's crust.
Chemical Composition of Geothermal Fluids
The chemical composition of geothermal fluids is complex and varies depending on several factors, including the source of the water, the types of rocks it has interacted with, the temperature and pressure conditions at depth, and the residence time within the geothermal reservoir. Major dissolved constituents typically include chloride (Cl-), sodium (Na+), potassium (K+), calcium (Ca2+), sulfate (SO42-), bicarbonate (HCO3-), and silica (SiO2). Trace elements such as lithium (Li), boron (B), arsenic (As), and antimony (Sb) are also often present, and their concentrations can provide valuable information about the source and evolution of the geothermal fluids. Gases such as carbon dioxide (CO2), hydrogen sulfide (H2S), methane (CH4), and ammonia (NH3) are also common components, and their presence can significantly influence the pH and redox potential of the fluid. The analysis of stable isotopes, such as deuterium (2H) and oxygen-18 (18O), can provide crucial insights into the origin of the water and the extent of water-rock interaction.
Origin of Geothermal Fluids
The origin of geothermal fluids can be traced to several sources, including meteoric water (precipitation), magmatic water (released from magma), and metamorphic water (released during metamorphic reactions). In most hydrothermal systems, meteoric water is the primary source, infiltrating the subsurface and being heated by contact with hot rocks. Magmatic water can contribute significantly in volcanic regions, particularly in systems associated with active volcanoes. The relative contributions of these different sources can be determined by analyzing the stable isotopes of the water. For instance, meteoric water typically has a distinct isotopic signature compared to magmatic water. The interaction of these waters with the surrounding rocks results in the dissolution of minerals and the acquisition of dissolved constituents, further modifying the fluid chemistry. Understanding the origin of geothermal fluids is crucial for developing conceptual models of geothermal reservoirs and for predicting their long-term behavior.
Geothermometry: Estimating Subsurface Temperatures
Chemical geothermometers are empirical or theoretical relationships between the concentration of certain dissolved constituents in geothermal fluids and the temperature at which the fluid equilibrated with the surrounding rock. These geothermometers are based on the principle that the solubility of minerals is temperature-dependent. By analyzing the concentrations of these constituents in the geothermal fluids, we can estimate the subsurface temperature of the geothermal reservoir. Several types of chemical geothermometers are commonly used, including silica geothermometers (based on the solubility of quartz or chalcedony), cation geothermometers (based on the ratios of Na/K, Na/Li, or K/Mg), and multi-component geothermometers (which consider the concentrations of multiple constituents). The choice of geothermometer depends on the specific geological setting and the chemical characteristics of the geothermal fluids.
Silica Geothermometers
Silica geothermometers are based on the principle that the solubility of silica minerals, such as quartz and chalcedony, increases with temperature. The concentration of dissolved silica in geothermal fluids is therefore an indicator of the temperature at which the fluid equilibrated with these minerals. Different silica geothermometers are used depending on the form of silica that is believed to have controlled the silica concentration in the fluid. Quartz geothermometers are typically used for higher-temperature systems, while chalcedony geothermometers are used for lower-temperature systems. However, it is important to consider factors such as mixing and dilution, which can affect the accuracy of silica geothermometers.
Cation Geothermometers
Cation geothermometers are based on the temperature-dependent exchange of cations between geothermal fluids and minerals. Common cation geothermometers include the Na/K geothermometer, the Na/Li geothermometer, and the K/Mg geothermometer. These geothermometers are based on the equilibrium constants for the exchange reactions, which are temperature-dependent. The Na/K geothermometer is particularly useful for high-temperature systems, while the K/Mg geothermometer is more sensitive to lower temperatures. However, these geothermometers can be affected by factors such as water-rock interaction and the presence of clay minerals, which can alter the cation ratios in the fluid.
The following table demonstrates how different geothermometers react to differing fluid compositions. This data illustrates that no single geothermometer is perfect; understanding the geological and chemical context is key to interpretation.
| Geothermometer | Formula Example (Simplified) | Temperature Range (°C) | Applicability | Limitations |
|---|---|---|---|---|
| Quartz (no steam loss) | T = 1309 / (5.19 - log(SiO2)) - 273.15 | 100 - 250 | Most common; Relatively reliable in many systems. | Affected by amorphous silica; May underestimate if rapid cooling occurs. |
| Na/K | T = 1217 / (log(Na/K) + 1.483) - 273.15 | 150 - 350 | Useful for high-temperature systems; Quick equilibration rates. | Sensitive to water-rock interaction; Less accurate in low-temperature or diluted systems. |
| K/Mg | T = 4410 / (14.0 - log(K²/Mg)) - 273.15 | < 200 | Useful for low-temperature systems; Sensitive to small changes. | Equilibrates very rapidly; Easily re-equilibrated during ascent, so may not reflect deep reservoir temperatures. |
| Chalcedony | T = 1032 / (4.69 - log(SiO2)) - 273.15 | < 180 | Applicable in some lower temperature areas where quartz is slow to equilibrate. | Less reliable than quartz at higher temperatures. Can be affected by chalcedony precipitation. |
Multi-Component Geothermometry
Multi-component geothermometry employs sophisticated geochemical modeling to account for multiple dissolved species and their interactions within geothermal fluids. This approach utilizes thermodynamic databases and software to calculate the equilibrium temperature based on the overall fluid composition. By considering the activities of various ions and complexes, multi-component geothermometry can provide more robust and accurate temperature estimates, particularly in complex hydrothermal systems where simple geothermometers may be unreliable. It also allows for evaluating the saturation indices of different minerals, helping to understand the potential for scaling and corrosion during geothermal energy extraction. It is an advanced method utilized extensively in geothermal exploration and management.
Applications in Geothermal Exploration and Reservoir Management
The analysis of geothermal fluids has numerous applications in geothermal exploration and reservoir management. In geothermal exploration, fluid geochemistry is used to identify promising areas for drilling and to assess the potential of geothermal reservoirs. The chemical composition of the fluids can provide information about the temperature, permeability, and extent of the reservoir. Chemical geothermometers are used to estimate the subsurface temperature and to guide the placement of exploration wells. In reservoir management, fluid geochemistry is used to monitor changes in the reservoir over time and to optimize the extraction of geothermal energy. Changes in the chemical composition of the fluids can indicate depletion of the reservoir, changes in the flow patterns, or the influx of cooler water. By monitoring these changes, operators can adjust the production strategy to maximize the long-term sustainability of the geothermal reservoir. This also has implications for environmental geochemistry, particularly in monitoring the release of trace elements during production.
Troubleshooting & Pro Tips
When analyzing geothermal fluids, it's crucial to handle samples with care to prevent contamination or alteration of their chemical composition. Immediately after collection, filter the samples to remove particulate matter and acidify them to prevent precipitation of dissolved constituents. Store the samples in airtight containers and keep them refrigerated until analysis. When interpreting geothermometer results, consider the geological context and the limitations of each geothermometer. Use multiple geothermometers and compare the results to obtain a more reliable estimate of the subsurface temperature. Be aware of potential sources of error, such as mixing, dilution, and water-rock interaction. Finally, always validate your interpretations with other geological and geophysical data.
FAQ
Q: What are the key parameters to measure in geothermal fluids?
A: The key parameters include pH, temperature, electrical conductivity, and the concentrations of major ions (Cl-, Na+, K+, Ca2+, SO42-, HCO3-, SiO2), trace elements (Li, B, As, Sb), and gases (CO2, H2S, CH4, NH3). Stable isotopes of water (2H and 18O) are also important.
Q: How reliable are chemical geothermometers?
A: The reliability of chemical geothermometers depends on several factors, including the accuracy of the analytical data, the geological setting, and the chemical characteristics of the geothermal fluids. No single geothermometer is perfect, and it is important to use multiple geothermometers and compare the results. Careful consideration of the geological context and potential sources of error is essential for accurate interpretation.
Q: What is the role of fluid geochemistry in geothermal exploration?
A: Fluid geochemistry plays a crucial role in geothermal exploration by providing information about the temperature, permeability, and extent of geothermal reservoirs. The chemical composition of geothermal fluids can be used to identify promising areas for drilling and to assess the potential of geothermal energy resources. Chemical geothermometers are used to estimate subsurface temperatures and to guide the placement of exploration wells. Understanding the water-rock interaction processes also provides insights into reservoir sustainability.